INTRODUCTION
This essay is about atoms. Every solid, liquid, gas and plasma are made of neutral or ionized atoms. Above is a picture of a helium atom, the pink is the nucleus and the black is the electron cloud, both of which I will go into in this essay.
Atomic explosions:

Atomic explosions are really big explosions. An atomic explosion happens when you split an atom. They look like this:
A 23 kiloton tower shot called BADGER, fired on April 18, 1953 at the Nevada Test Site, as part of the Operation Upshot–Knothole nuclear test series.
The nucleus
The atomic nucleus is in the center of an atom. It is made of protons and neutrons, and was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg.
Dmitri Ivanenko
Ukrainian physicist-theorist Dmitri Ivanenko, made a great contribution to the physical science of the twentieth century, especially to nuclear physics, field theory, and gravitation theory.
Werner Heisenberg
Werner Heisenberg was a German theoretical physicist and one of the key pioneers of quantum mechanics.
Electron cloud
An atomic orbital(or electron cloud) is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom’s nucleus. The term, atomic orbital, may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.(gesundheit)
CONCLUSION
I hope this makes sense and encourages you to learn about science, physics and other subjects.
Thank you.
Special thanks to wikipedia for the information in this essay.
1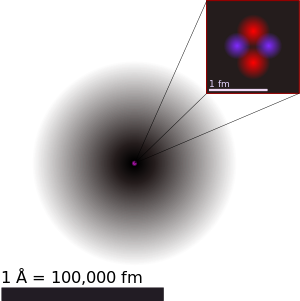
You must be logged in to post a comment.